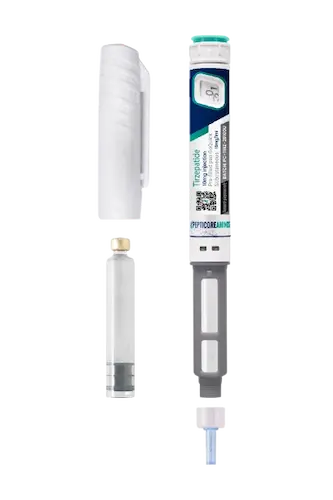
Tirzepatide
Dual GIP/GLP-1 peptide for research on diabetes, obesity, and metabolism.
What is Tirzepatide
Tirzepatide is a next-generation peptide molecule developed for the treatment of type 2 diabetes and obesity. It is the active compound in the commercial drug Mounjaro, approved worldwide as a dual agonist of the GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors. This combination of hormonal targets allows simultaneous action on blood glucose regulation, lipid metabolism, and appetite control.
Unlike previous GLP-1 agonists, Tirzepatide produces a synergistic effect that improves glycemic control, promotes significant and long-lasting weight loss, and enhances the body’s overall energy efficiency. In subjects with obesity, treatment with Tirzepatide has shown body weight reductions of up to 22.5% over 72 weeks, as reported in the reference study SURMOUNT-1 (NEJM 2022).
Mechanism of Action: synergy between GIP and GLP-1
Tirzepatide mimics the combined action of two natural intestinal hormones, GIP and GLP-1, known as incretins. These hormones are secreted after meals and contribute to the regulation of glucose and fat metabolism. By acting on both receptors, Tirzepatide amplifies the signals that control insulin secretion, reduces glucagon levels, and modulates the brain centers of appetite.
GIP Receptor
The GIP receptor stimulates the pancreas to release insulin only when blood glucose levels are high, minimizing the risk of hypoglycemia. It also participates in fat metabolism by influencing the use of fatty acids as an energy source. In combination with GLP-1, this pathway promotes the reduction of visceral fat, the type most closely associated with cardiovascular risk and insulin resistance.
GLP-1 Receptor
Activation of the GLP-1 receptor reduces hunger, slows gastric emptying, and stimulates insulin secretion in response to glucose. At the same time, it inhibits the release of glucagon, thereby lowering blood sugar levels. The combined effect improves HbA1c, stabilizes fasting glucose, and facilitates the maintenance of a prolonged caloric deficit.
This dual endocrine action underlies the success of Tirzepatide, combining the antidiabetic properties of GLP-1 with a broader metabolic impact mediated by GIP. Clinical results also suggest a potential role in improving vascular health, reducing blood pressure, and lowering systemic inflammatory markers
Main Observed Benefits
- Optimized glycemic control through enhanced glucose-dependent insulin secretion.
- Sustained weight loss and reduction of visceral fat mass.
- Improved lipid profile with lower LDL cholesterol and higher HDL.
- Reduced appetite and prolonged post-meal satiety.
- Positive effects on energy metabolism and cardiovascular function.
Comparison with Other Incretin Agonists
Tirzepatide belongs to the same pharmacological class as Semaglutide and the newer Retatrutide, but differs in the number of receptors activated and the intensity of the metabolic effect. Comparative studies have shown that Tirzepatide outperforms Semaglutide in improving glycemic control and achieving greater weight reduction, as demonstrated in the SURPASS-2 (NEJM 2021) trial.
| Drug | Activated Receptors | Main Effects | Average Weight Loss (Clinical Data) |
|---|---|---|---|
| Semaglutide | GLP-1 | Satiety, slower gastric emptying, glycemic control | ≈ 15% in 68 weeks |
| Tirzepatide | GLP-1 + GIP | Dual action on appetite, glucose, and lipid metabolism | ≈ 22,5% in 72 weeks(SURMOUNT-1) |
| Retatrutide | GLP-1 + GIP + glucagon | Higher energy expenditure and fat oxidation | ≈ 24% in 48 weeks(Phase 2, preliminary data) |
Scientific References
Jastreboff AM et al. Tirzepatide Once Weekly for the Treatment of Obesity (SURMOUNT-1). N Engl J Med. 2022.
Frías JP et al. Tirzepatide versus Semaglutide Once Weekly in Type 2 Diabetes (SURPASS-2). N Engl J Med. 2021.
Krumholz HM et al. Blood Pressure and Cardiometabolic Effects of Tirzepatide: Analyses from SURMOUNT-1. 2024.
American College of Cardiology – Clinical summary of SURPASS-2.
Notes and Warnings
The Tirzepatide supplied by Pepticore Aminos is intended for research and laboratory use only. It is not approved for human or animal consumption and must not be used as a medicinal or diagnostic product. All information is provided for research and educational purposes only and is based on publicly available clinical data.