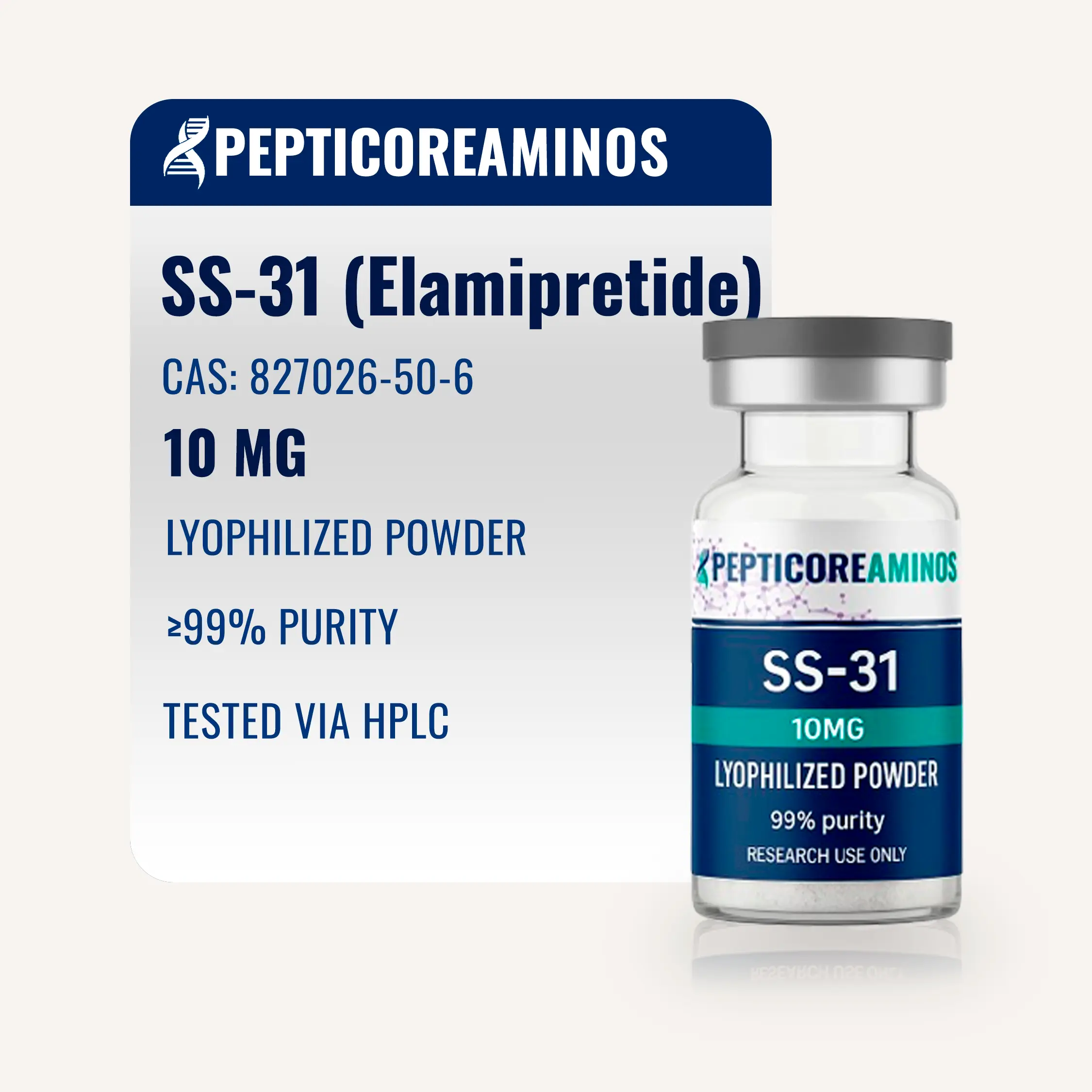
SS-31 (Elamipretide)
Synthetic tetrapeptide for research on mitochondrial function, cellular energy production, and protection from oxidative stress.
What is SS-31
SS-31, also known as Elamipretide or the Szeto-Schiller peptide, is an innovative synthetic peptide composed of four amino acids. It was developed around the year 2000 by researchers Hazel H. Szeto and Peter W. Schiller. Initially designed as a ligand for the μ-opioid receptor, it later revealed an unexpected ability to selectively accumulate in mitochondria, where it interacts with cardiolipin, an essential phospholipid found in the inner mitochondrial membrane.
This interaction allows SS-31 to stabilize mitochondrial structure, improve the efficiency of ATP production (the main source of cellular energy), and reduce the formation of reactive oxygen species (ROS), the main culprits behind cellular damage and aging. Thanks to its small size and lipophilic properties, the peptide easily crosses cell membranes and reaches mitochondria in various tissue types.
SS-31 and mitochondrial function
Mitochondria are the cell’s power plants, responsible for producing ATP through oxidative phosphorylation. However, when mitochondrial function deteriorates—due to aging, oxidative stress, or genetic mutations—cells lose energy and begin to degenerate. SS-31 binds to cardiolipin, stabilizing the cristae structure, improving cellular respiration, and limiting lipid peroxidation.
Early experimental evidence showed that SS-31 accelerates ATP recovery and reduces necrosis in animal models of ischemia-reperfusion—a process in which blood and oxygen flow are temporarily interrupted and then restored, causing severe cellular damage. In studies on aged mice, a single dose of SS-31 rapidly improved mitochondrial bioenergetics, increasing ATP production and reducing oxidative stress. These results were not observed in young subjects, indicating that the peptide acts selectively on age-related mitochondrial deficits.
Applications in biomedical research
Mitochondrial dysfunction is implicated in numerous chronic and degenerative diseases, including Alzheimer’s, Parkinson’s, metabolic syndrome, cardiomyopathies, and mitochondrial myopathies. SS-31 is currently the subject of clinical studies as a potential therapy for primary mitochondrial myopathies and heart failure. In preclinical experiments, the peptide has shown the ability to reduce mitochondrial permeability transition pore (MPT) opening, preventing mitochondrial depolarization and apoptosis. These protective effects, combined with reduced inflammation and lower radical production, make it a highly promising candidate in mitochondrial pharmacological research.
Moreover, SS-31’s ability to preserve mitochondrial integrity translates into improved muscle endurance, reduced oxidative damage, and better cardiac and renal function in models of ischemia and energy deficiency.
SS-31 and cardiac protection
Mitochondrial dysfunction plays a key role in heart failure and ischemic injury to the myocardium. SS-31 has emerged as a potential cardioprotective agent, capable of restoring mitochondrial function and limiting damage caused by ischemia and oxidative stress. Studies on human cardiac tissues have shown that SS-31 increases mitochondrial oxygen flux and enhances the activity of respiratory complexes without altering cardiolipin structure. These results suggest a multiple mechanism of action that goes beyond simple lipid stabilization.
In animal models of advanced heart failure, chronic treatment with SS-31 improved left ventricular function and increased ATP production, slowing cardiac tissue remodeling. In studies on acute myocardial infarction (STEMI), the peptide significantly reduced cardiomyocyte apoptosis and tissue damage spread, confirming its potential role in heart protection during ischemic events.
SS-31 and glucose metabolism
Mitochondrial dysfunction is one of the main causes of oxidative stress associated with type 2 diabetes. SS-31 has been shown to reduce ROS production in metabolically active tissues, improve mitochondrial membrane potential, and increase levels of SIRT1, a protein linked to insulin sensitivity and cellular longevity.
In both clinical and preclinical studies, SS-31 treatment led to a decrease in inflammatory markers such as NFκB-p65 and TNFα, as well as improved interactions between leukocytes and endothelial cells. These combined effects suggest a potential role for the peptide in enhancing mitochondrial function and preventing cardiovascular and microvascular complications associated with diabetes.
SS-31 and inflammation modulation
SS-31 also acts as a modulator of chronic inflammation through its ability to neutralize reactive oxygen species and protect mitochondrial structure. By reducing the expression of pro-inflammatory proteins such as CD36, FIS1, and NF-κB p65, the peptide helps maintain mitochondrial morphology and prevents activation of the inflammasome, responsible for many cellular inflammatory responses.
At the same time, SS-31 enhances endogenous antioxidant defense mechanisms such as MnSOD and catalase, reducing the production of inflammatory cytokines like TNF-α. In animal models, SS-31 treatment has been shown to reduce tissue damage and improve histological parameters in various organs, including the kidney and brain, strengthening its potential in combating chronic inflammation and degenerative diseases.
Perspectives and clinical development
Although phase III clinical trials have not yet provided conclusive results, phase II studies have shown significant improvements in physical performance and bioenergetic parameters in patients affected by primary mitochondrial myopathy. SS-31 has demonstrated an excellent safety profile and good tolerability, with no significant adverse effects reported.
Researchers such as Bruce Cohen have noted that the failure of later phases may be due to suboptimal endpoint selection and that future studies with more targeted protocols could confirm the peptide’s therapeutic potential. Currently, new clinical studies are underway to test its efficacy in various conditions, including Barth syndrome, age-related macular degeneration, and neurodegenerative diseases.